Health Technology Assessment (HTA) is a relatively new system to Japan, resulting in a low level of awareness in the society. As an inseparable piece of patient-centered care, especially for managing chronic diseases like diabetes that require long-term treatment, a thorough understanding in HTA, including its cost-effectiveness evaluation system, is crucial.
What is Health Technology Assessment (HTA)?
Health technology assessment (HTA) is a comprehensive evaluation of health technologies, taking account of medical, economic, and social impacts. However, the definition of HTA varies widely, from the evaluation itself to the research used to determine drug reimbursement and pricing.
Given the goal of HTA is to maintain insured medical treatment and optimize the healthcare system, its achievement requires a thorough examination of health technologies from all aspects, including health outcomes and costs. As an example, a drug’s cost-effectiveness is assessed by comparing its cost to that of standard treatment, along with factors like survival rate and QALY (Quality-adjusted Life Year).
The growing need to assess the aspect on health economics of chronic diseases, such as diabetes, has brought HTA into greater focus.
Expectation on HTA: Recent Diabetes Treatment in Japan
Knowing how diabetes has been recently treated in Japan will help capture the background of the growing needs in HTA. In 2016, the Japanese Ministry of Health, Labour and Welfare (MHLW) estimated that approximately 20 million people have diabetes in Japan, including those at risk, meaning one in ten people aged 20-64 is subject to this condition. (*)
Long-term treatment is essential to achieve the goal of diabetes treatment, including QoL (Quality of Life) and life expectancy comparable to a healthy person. In addition, numerous other diseases require long-term treatment, which will eventually coerce further financial burden on patients and society. Consequently, it underscores the increasing importance of HTA.
*Source: Ministry of Health, Labour and Welfare, 2018 White Paper on Health and Welfare – Addressing Disabilities, Illnesses, Etc., and Creating a Society Where Everyone Can Play an Active Role – (Accessed July 26th, 2023)
An Overview of Cost-Effectiveness Evaluation in HTA
Around 2012, Japan started to consider implementing a cost-effectiveness evaluation system in HTA. The formal establishment of the system is executed by the MHLW in 2019 upon completing a pilot program from 2016. Although it is often interchangeable with cost performance in Japan, cost-effectiveness in HTA has a distinct meaning. In HTA, cost-effectiveness evaluation compares new health technologies and drugs to existing treatment or drugs, based on the perspectives of cost and effectiveness.
Selection Criteria for Target Products
Cost-effectiveness evaluations are applied to innovative drugs that have significant financial impact. These drugs are categorized into H1 to H5 classifications based on drug pricing methods and selection criteria. Although the cost-effectiveness evaluation system only covered a limited number of products since its inception, the number of covered products is on the rise.
The MHLW defines the selection criteria as follows: (*1)
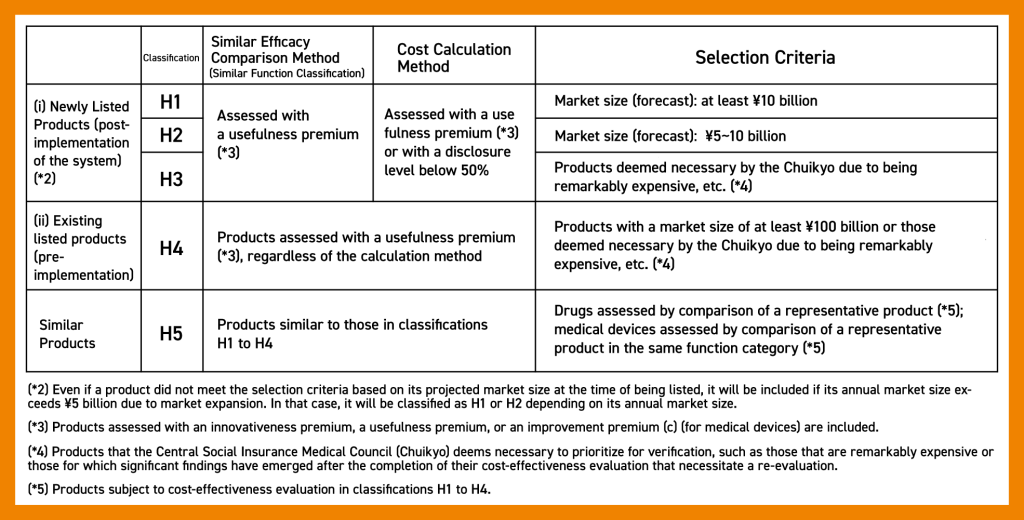
(*1) Source: Ministry of Health, Labour and Welfare, Selection Criteria for Products Subject to Cost-Effectiveness Evaluation (Accessed July 14th, 2023)
Evaluation Method
The cost-effectiveness evaluation method is mainly based on quality-adjusted life year (QALY), a numerical value that multiplies quality of life by years of survival, and incremental cost-effectiveness ratio (ICER). The incremental cost-effectiveness ratio (ICER) is a statistic used in cost-effectiveness analysis to summarise the cost-effectiveness of a health care intervention. It is defined by the difference in cost between two possible interventions, divided by the difference in their effect. It represents the average incremental cost associated with 1 additional unit of the measure of effect, often described in monetary units, while effects can be measured in terms of health status or another outcome of interest. A common application of the ICER is in cost-utility analysis, in which case the ICER is synonymous with the cost per quality-adjusted life year (QALY) gained.
Using a threshold of ¥5 million/QALY (with an upper limit of ¥10 million/QALY), if the calculated ICER exceeds this value, the price will be adjusted downwards in stages. Although this method is the same as that used in other countries that have introduced cost-effectiveness evaluation systems, the threshold value is unique to Japan.
There was discussion on calculating the threshold, which serves as the matrix of the evaluation method, based on GDP per capita and willingness-to-pay survey, but opinions are divided. Additionally, the appropriateness of the threshold is gaining attention among professionals, concerning the increase of expensive drugs and medical treatments in the future.
Cost-Effectiveness Evaluation: Framework and Process
The process begins with a thorough analysis and evaluation of the product in question, requiring approximately one to one and a half years for completion. A final comprehensive evaluation and pricing decision follow. (*) The final evaluation incorporates various perspectives in addition to the ICER value.
The National Institute of Public Health established the Center for Health Economics Research and Evaluation to oversee these evaluations. However, several shortcomings are still hidden in this system, notably the insufficient incorporation of patient perspectives.
Reforms to HTA’s cost-effectiveness evaluation were implemented in 2022, including revisions to some processes and analysis periods. The evaluation system and process are expected to evolve significantly as further revisions are anticipated.
Source: Ministry of Health, Labour and Welfare, About the Cost-Effectiveness Evaluation System, (Accessed July 14th, 2023)
The Importance of HTA in Patient-Based Healthcare
Delivering patient-centered care has a strong reliance on HTA. Medical and economic evaluations are especially crucial for chronic diseases like diabetes, which require long-term treatment. Although HTA is a relatively new system in Japan, the significance of HTA within the Japanese healthcare system is expected to grow, resulting in high expectations on realizing a more effective system through continued refinement in the future.




