Side effects caused by medicines: Explanation of response methods and patient side effect reporting system
While medicines help cure illnesses and injuries, or alleviate and prevent symptoms, they often have side effects. The number of reports continues to increase year by year in Japan as well, and according to data from the Ministry of Health, Labor and Welfare’s Pharmaceutical and Living Hygiene Bureau in 2019, the number of reports from pharmaceutical manufacturers and distributors reached 60,405 events in a year.
In this article, we will explain the types and symptoms of side effects, what to do when they occur, and an overview of patient side effect reports that were required by the Pharmaceuticals and Medical Devices Agency (PMDA) as of March 2019.
Regarding Drugs’side-effects
By defining “side effects”, a drug has a main effect of healing an illness or injury, but it may have a different effect, which is called a side effect.
For example, if you take a medicine for hay fever, your runny nose and itching in your eyes may have subsided, but you may suffer from sudden drowsiness. That is what we call a side-effect.
Side effects can be broadly divided into the following four types of symptoms.
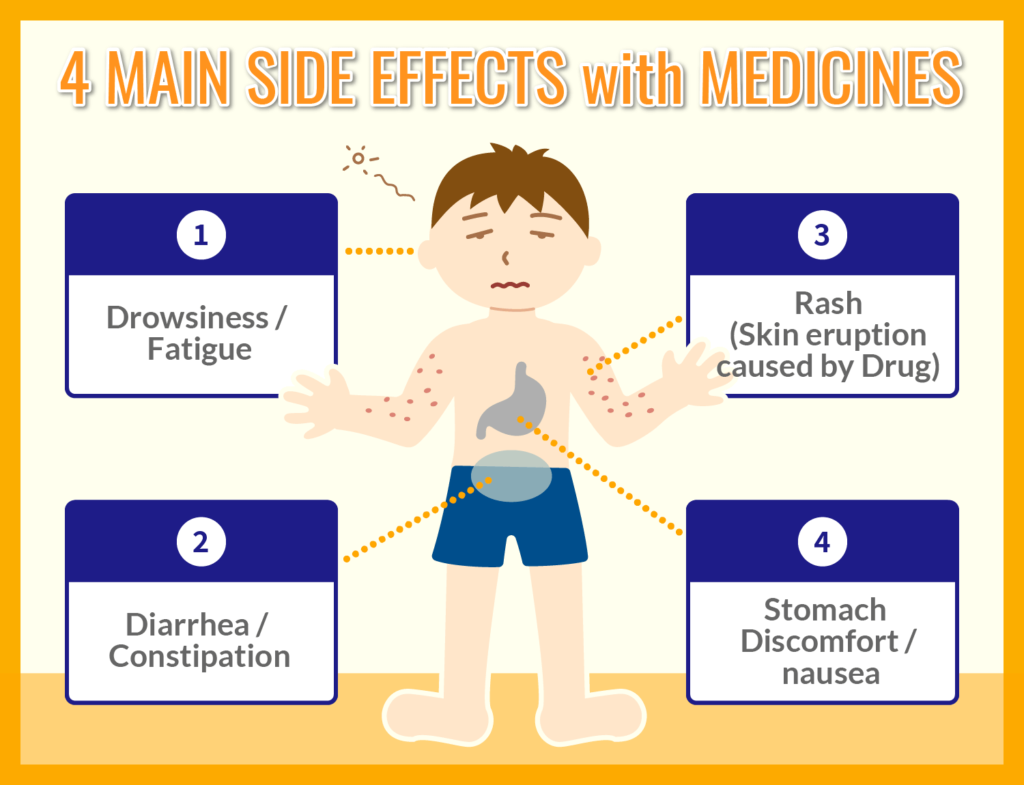
- Drowsiness / fatigue
When taking oral medicines such as cold medicines and pollinosis medicines to control allergies, and painkillers for stomach and abdominal pain, symptoms such as drowsiness and malaise tend to occur in many cases. - Diarrhea / constipation
Since oral medications are absorbed in the intestinal tract, diarrhea and constipation often occur as side effects. For example, antibiotics that are often prescribed for the treatment of skin diseases often have diarrhea as a side effect, and anticholinergic drugs contained in antidepressants often cause constipation as a side effect. - Rash ( Skin eruption )
There are many cases in which an allergic reaction to a drug occurs and a rash (skin eruption) appears as a side effect.
Typical side effects include redness, urticaria, chickenpox, and abnormal itching of the skin. In some cases, it occurs immediately after using the drug, in other cases it occurs 1 to 2 weeks later, and in some other cases it may occur more than 1 month later. - Stomach discomfort / vomiting
Taking cold medicines, antipyretic analgesics, or medicines containing antibiotics may cause stomach upset, which may cause side effects such as continued discomfort and vomiting. These side effects are more likely to occur if the drug is used without adhering to the prescribed dosage and administration.
Categories of Side-Effect
There are various appearances and causes of side effects, but they can be roughly divided into three types.
- Side effects based on pharmacological action
The therapeutic effect of the drug results from the strict observance the correct dosage and administration for the indicated disease. Improper use tends to cause side effects.
For example, in the case of stomach discomfort and vomiting (nausea) mentioned above, when a cold medicine or antipyretic painkiller is taken on an empty stomach, the stomach misunderstands the medicine as food and secretes gastric acid, causing the gastric mucosa to become irritated. It is a side effect that is more likely to occur.
In addition, many medicines are metabolized in the kidneys and liver, so when taken by people with abnormal functions, blood levels may continue to be high and adverse side effects may occur.
In addition, the ability to metabolize medicines tends to decline with age, and older people are more likely to have side effects than younger people, even if they follow proper usage. - Side effects affected by constitution
The medicine does not contain any ingredients that have a strong adverse effect on the human body but depending on the constitution of the person taking the medicine, the ingredients of the medicine may cause an allergic reaction.
Side effects due to constitution appear even if they are used according to the correct usage and dosage, and typical symptoms include the above-mentioned rash (skin eruption). It can also cause anaphylaxis if the allergic reaction is severe.
We will explain anaphylaxis in detail later. - Drug interaction
In recent years, while medical care has become more sophisticated, the aging of the population has also progressed. Many people have multiple illnesses and visit various clinical departments, and there are a lot of cases where different types of medicines are used at the same time. Under such circumstances, the risk of drug-drug interactions due to multiple doses and polypharmacy is increasing.
There are many cases in which each department is unable to keep track of the drugs prescribed by other departments, and drugs with harmful drug interactions are prescribed. As a result, it often causes serious side effects.
Regarding Anaphylaxis
Anaphylaxis is a term that refers to a particularly serious condition in terms of allergic reactions caused by drugs side effects.It is defined as “hypersensitivity reaction that can be life-threatening due to systemic allergic symptoms.”
Anaphylaxis appears systemically within minutes to tens of minutes of the drug absorption in the body.
The following shows the typical symptoms of anaphylaxis.
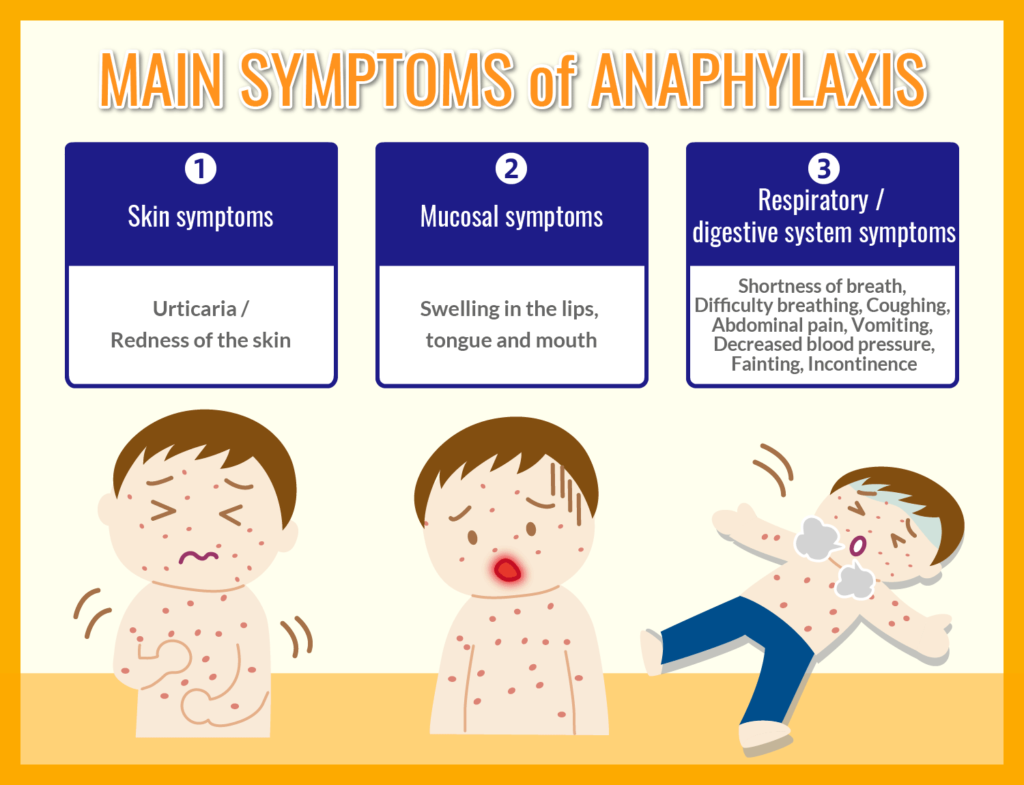
In addition, when blood pressure drops down to level of impaired consciousness, it is called “anaphylactic shock”. Anaphylactic shock can be a life-threatening condition unless treated immediately at a medical institution.
The drugs that are likely to cause anaphylaxis are:
- Antibiotic agents
A majority of the side effects are reported with antibiotics called β-lactams, and there are also reports of side effects with antibiotics such as new quinolones. - Antipyretic analgesic
NSAIDs (= Non-Steroidal Anti-Inflammatory Drugs) are abundant in well-known over-the-counter drugs such as loxoprofen and ibuprofen.Many side effects have been reported. - Local anesthetics
It is said that anaphylactic shock is often a response to additives and vasoconstrictors contained in local anesthetics. - Anti-tumor drugs
Platinum preparations, taxanes, etc. - Contrast agents
Contrast media used in CT and MRI examinations are said to cause anaphylaxis with a probability of 1 in thousands. - Muscle relaxant
It is said to be the most common cause of anaphylaxis that occurs during general anesthesia. - Blood transfusion product
Many side effects due to red blood cell preparations or platelet preparations have been reported.
Anaphylaxis is an allergic reaction that can occur not only with medicines but also with food (eggs, milk, wheat, etc.) and insect bites such as bees.
What to do when side effects occur
The following are the measures to be taken when unusual symptoms appear using medicines or when symptoms that seem to be side effects occur.
Consulting a doctor or a pharmacist
If you experience symptoms that appear to be side effects of your medicine, you should stop using the medicine and consult your doctor / pharmacist for instructions as a primary response.
Depending on the side effects that appear, the response will change, such as stopping the use of the drug, continuing to use it while watching the situation, reducing the dose of the drug, changing the drug, and so on.
Also, depending on the type of drug, it may be more disadvantageous for the patient to stop using it even if side effects appear. The above judgment should not be made by the patient but should be left to the doctor or pharmacist who is a drug expert.
In any case, it is important that each patient be able to clearly explain “which medicine”, “how much has been used”, “how long has been the treatment with the medicine”, and “what kind of symptoms occurred when” to ensure the optimal coordination with healthcare professionals.
Consulting via call center and internet websites
If the side effects are mild, or if your medical institution is closed or you cannot immediately consult a doctor or pharmacist at night, you can check the consultation desk on the Internet or use call centers for consultation.
The website of the Japan Pharmaceutical Association introduces a list of consumer medicine consultation counter names nationwide.The website of the Japan Pharmaceutical Manufacturers Association (JPMA) provides a list of consumer medicine consultation counters for member companies (pharmaceutical companies).
PMDA accepts telephone consultations from 9am to 5pm, Monday to Friday.
In addition, PMDA’s homepage has a content called a drug guide for patients, which clearly describes precautions and side effects when using various drugs, and if the drugs or ingredients could be suspected of causing side effects? It is a useful reference to evaluate if the side-effect has been observed or not.
How to use the Relief Fund for Drug side-effects
As mentioned above, medicines may cause unexpected side effects even if they are used correctly and in the correct dosage and administration. There is a damage relief system that allows patients to receive benefits such as medical expenses coverage if serious side effects occur even though the drug has used properly according to the instruction manual (package insert).
This system is a public system established for the purpose of prompt relief for people who have suffered health damage, and serious health damage caused by the side effects of medicines used after May 1, 1980 (Showa 55). It provides medical expenses and disability pensions for aftereffects when hospitalization is required. It was founded after the pharmacological toxicity incidents such as thalidomide and SMON that became a social problem in the 1960s.
However, this relief system does not cover all health hazards caused by the side effects of medicines. The Pharmaceutical Affairs and Food Sanitation Council will make a medical and pharmaceutical decision before deciding whether or not to provide the benefits.This system does not cover quasi-drugs and cosmetics that are on the market.
The following three cases are eligible for benefits.
- Cases in which severe symptoms were confirmed that required hospitalization
- Cases in which severe symptoms were confirmed that significantly restricted daily life
- A case in which a patient died due to side effects.
To receive the benefits, it is necessary that the application is made by the person who suffered health damage or his / her family. They will send the invoice and the documents necessary for the invoice (medical certificate by doctor, sales certificate by pharmacy / drugstore, etc.) to PMDA and make the claim for compensation.
There are seven types of benefits, such as medical expenses and medical allowances, and each has a different billing deadline and required documents.
Detailed information on the application procedure can be found on the PMDA website.
Reporting patient’s side-effect
Patient side effect reporting is an initiative to collect side effect reports from patients and their families widely for ethical drugs and over-the-counter drugs manufactured and sold in Japan and utilize the data for safety measures.
In the past, side effect reports were mainly reported by medical personnel and pharmaceutical companies, but it is necessary to have a side effect reporting system from general patients, which was first implemented in Western countries as an initiative to prevent recurrence in the wake of the drug-damaged hepatitis incident. In Japan, the patient side effect reporting system was established on the PMDA website in March 2012, and the acceptance of side effect reports from patients or their families began on a trial basis.
From March 26, 2019 (Heisei 31), the scheme officially started on the PMDA homepage, and there is an expectation that it will be useful for understanding the side effects of drugs that were unknown until now and for using the drugs more safely.
The content of reporting
The items to be reported to PMDA in the patient side effect report are as follows.
- Patient / reporter information
Name / age / gender / address / height / weight / etc - Information on drugs that may have caused symptoms with suspected side effects
Name of drug / start date and time of use / end date of use / purchase information / dosing status after side effects appear, etc. - Information about side effect symptoms
Symptoms of side effects / Time of onset / Whether or not to consult a medical institution after the onset of side effects / Recovery status / Illnesses that have occurred before the side effects appear, surgery and timing that have been performed, etc. - Information about medical institutions where you can hear detailed information
Name of medical institution / phone number / name of attending physician, etc.
Data reported by patients and their families will be checked by PMDA personnel to see if they are already recognized side effects, and then further safety measures will be considered.
How to perform the reporting
Patient side effect reports are basically sent by filling in the required items in the report form on the PMDA homepage. They also accept reports by mail, if patients print the report format from the PMDA homepage. It is possible to request the report format by telephone from the PMDA patient side effect report form request window, fill in the necessary items, and send it to the PMDA Safety Information and Planning Management Department. Completion of the report by mailing also a possibility.
Conclusion
Reporting side effects is an obligation stipulated in the Pharmaceutical & Medical Device Act for any company that manufactures and sells pharmaceuticals, quasi-drugs, cosmetics, and medical devices. Therefore, if there is a report omission or delay, authorities would immediately release a business improvement order. In order to prevent omissions and delays in reporting side effects, it is necessary to understand as much as possible the side effect data and the probability of onset of drugs-drugs interactions from the research stage up to manufacturing development.
Medical Data Vision Co., Ltd. can provide aggregated results that can be used as reference values for side effects of drugs.
If you have any problems in collecting information and data related to side effects, please feel free to contact us.
For More Information, Please Contact Us Here
About Japanese Healthcare System

What you need to know about the healthcare system in Japan before using the data.
SERVICE

In addition to various web tools that allow you to easily conduct surveys via a browser using our medical database, we offer data provision services categorized into four types to meet your needs and challenges: "Analysis reports" "Datasets," "All Therapeutic Areas Data Provision Service," and "Specific Therapeutic Areas Data Provision Service.

© Medical Data Vision Co., Ltd. All Rights Reserved.





