What is Post-Marketing Surveillance (PMS)?
A complete description of the 3 different types of PMS, how it differentiates from early-phase pharmaco-vigilance investigation (EPPV) and the overall flow of data
This article will explain the concept of “Post-Marketing Surveillance”.
The term “Post-Marketing surveillance” may be unfamiliar to some and it may be difficult to understand its specific meaning and procedures, but this article will give you an overview of the process.
This article will provide a detailed explanation of post-marketing surveillance, including an overview, purpose, types, flow, and differences from immediate post-marketing surveillance.
Contents
- What is post-marketing surveillance?
- Three main types of post-marketing investigations
- The flow of post-marketing investigations
- The necessity of a supervisor in post-marketing surveillance
- The retention period for post-marketing surveillance
- Differences between post-marketing surveillance and early-phase pharmaco-vigilance process
- Issues in post-marketing surveillance
- Please contact us when conducting surveys using medical information databases
What is post-marketing surveillance?
Post-Marketing Surveillance (PMS) is a method of monitoring the safety of drugs and medical devices after they have been approved for marketing and used in the general population, as well as additional indications are added.
Under the direction of the Ministry of Health, Labour and Welfare (MHLW), marketing authorization holders (pharmaceutical companies and medical device manufacturers) conduct surveys of medical institutions (hospitals and clinics) and report their findings to the MHLW.
Purpose of post-marketing surveillance
The purpose of post-marketing surveillance is to confirm the efficacy and safety of products. Additionally, due to the limited amount of information that can be obtained before the release of new drugs post-marketing surveillance is also used to collect usefulness indicators and long-term data.
Post-marketing surveillance has the following advantages.
- It can improve the safety of a drug or medical device by leveraging the sheer quantity of data used on the market.
- Much more information is available than in the clinical study phase.
Conducting post-marketing surveillance
The approved marketing authorization holders are obliged to reexamine the drug to confirm its efficacy and safety by investigating its use for eight years in general. However, post-marketing surveillance is part of the “investigation on the results of use”, and other surveillance methods are also available. Post-marketing investigation is conducted when it is necessary.
Three main types of post-marketing investigations
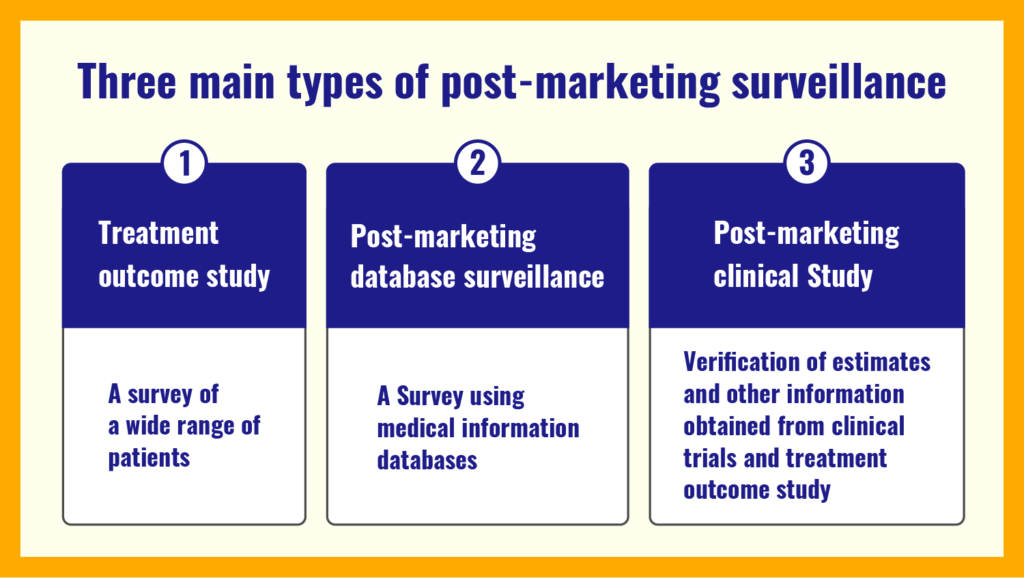
There are three main types of post-marketing investigations.
- Treatment outcome study
- Post-marketing database investigation
- Post-marketing clinical study (aka Phase IV study)
Each will be described in detail.
1. Treatment outcome study
Treatment outcome studies are investigations of the quality, efficacy, and safety of a new drug in a wide range of patients after its marketing launch.
The main purposes of treatment outcome studies are to discover and study unknown side effects and to identify factors that may affect safety and efficacy.
Furthermore, among treatment outcome studies, the following types of patients can be targeted, and such studies are called specific treatment outcome studies.
- Children
- The elderly
- Pregnant or post-pregnant women
- Persons with liver or kidney dysfunction
- Long-term users
2. Post-marketing database investigation
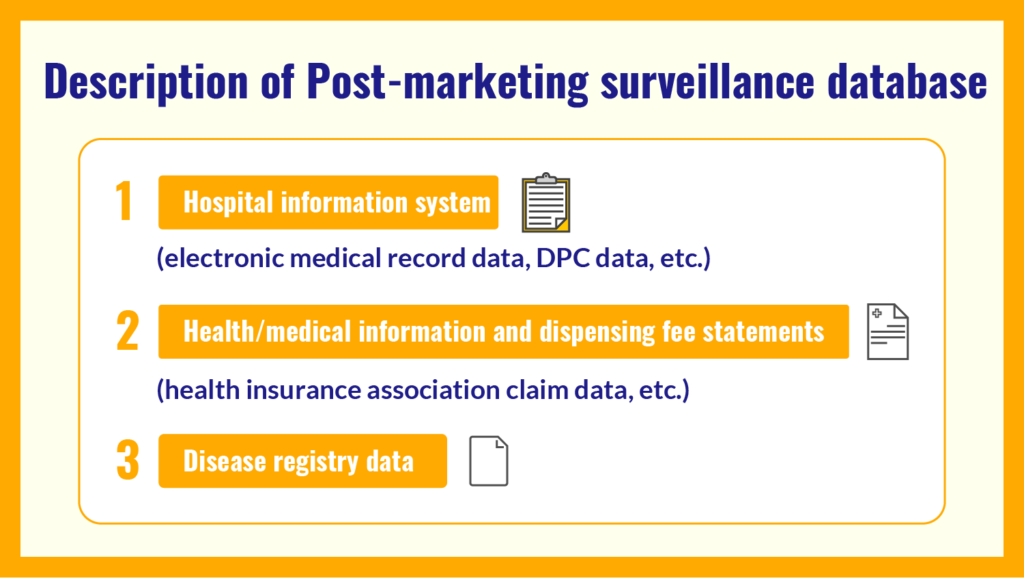
Post-marketing surveillance database is conducted to confirm the efficacy and safety of pharmaceutical products and to detect the occurrence of diseases caused by adverse reactions, using medical information data such as hospital information system data (electronic medical record data, DPC data, etc.), health / medical information and dispensing fee statements (health insurance association claim data, etc.), and disease registry data.
For example, a survey could focus on these items.
- What type of patients is it prescribed for?
- Are there any adverse events?
- Is the frequency of adverse events increasing compared to similar drugs?
- Is the amount of administration for patients with contraindications decreasing?
3. Post-marketing clinical study
Post-marketing clinical studies verify the results of clinical trials or treatment outcome studies.
The reason why post-marketing clinical studies are conducted is that in actual medical practice, drugs and medical devices are sometimes used under different conditions from those in the clinical trial phase, and effects or side effects that were not discovered in the clinical trials may be discovered.
Thus, drugs and medical devices are continuously investigated even after they are placed on the market. The results are reported to the MHLW for review again.
The flow of post-marketing investigations
Post-marketing surveillance is conducted in the following sequence.
- Development of implementation plan
- Periodic safety reporting
- Reexamination and reevaluation
Each will be described in detail.
1. Development of study implementation plans
Before conducting post-marketing surveillance, the following plans are developed to determine the necessity of the study.
- Safety surveillance plan: a plan to collect information mainly on side effects
- Risk minimization plan: a plan to minimize specific risks
Specifically, this includes the preparation of post-marketing surveillance reports, packaging inserts, drug guides, and immediate post-marketing surveillance.
These practices will help to clarify the purpose and necessity of the survey, preventing the random implementation of surveys whose purpose are unclear.
2. Periodic safety reporting
Once post-marketing surveillance is approved, investigations are conducted, and reports are made on side effects or infections.
Safety reports will be made regularly through treatment outcome studies, post-marketing database studies, and post-marketing clinical studies.
3. Reexamination and reevaluation
After the safety report is completed, the pharmaceutical company reconfirms and reevaluates the quality, efficacy, and safety on the market within the reexamination period specified after the release of the product. The investigation period is four to ten years.
Furthermore, the MHLW can reconsider the validity of a new drug as a reevaluation after reexamination.
The necessity of a supervisor in post-marketing surveillance
Post-marketing surveillance requires a “supervisor” who is responsible for overseeing the operations.
The supervisor must not belong to a department related to sales. They are responsible for the following tasks.
- Planning, drafting, and coordinating the survey
- Confirmation that the survey is being conducted properly and smoothly in accordance with the work procedures, plans, etc.
- Reporting results
- Revision of documents as necessary
Regular self-inspections of information gathering and operational procedures in post-marketing surveillance operations are also mandated.
The retention period for post-marketing surveillance
Post-marketing surveillance has a retention period, and the medical institution that conducted the surveillance must retain information and records related to the surveillance until the day when the reexamination or reevaluation by the MHLW is completed.
For medical institutions that will not move to a post-marketing clinical study, the retention period is until the later of either the date of approval for manufacturing / marketing or the date three years have passed after the discontinuation or termination of the clinical trial.
For example, if a medical institution conducting a clinical trial does not conduct a post-marketing clinical study as an additional investigation, the retention period is three years from the end of the clinical trial.
Differences between post-marketing surveillance and early-phase pharmaco-vigilance process
Immediate post-marketing surveillance has a similar concept to post-marketing surveillance.
This section explains the differences between post-marketing surveillance and immediate post-marketing surveillance.
What is early-phase pharmaco-vigilance (EPPV) procedure?
EPPV is a survey conducted during the first six months of sales to confirm the safety and efficacy of a new drug, with the following objectives.
- Promote proper use of new drugs: Provide reliable information and warnings to medical institutions to promote understanding of the proper use of new drugs.
- Minimize damage from side effects: Minimize damage from side effects by promptly collecting information on serious side effects or infections and implementing necessary safety measures.
This is part of the “Risk Management Plan (RMP)”, a measure to appropriately manage the risks of new drugs from the development stage to the post-marketing period, which is performed when considered necessary.
Hospitals and clinics are the main targets of immediate post-marketing surveillance. In some cases, pharmacies may also be covered.
EPPV has made it possible to identify potentially serious side effects at an early stage. As a result, the survey is now essential for maintaining the overall efficacy and safety of drugs.
Difference between post-marketing surveillance and immediate post-marketing surveillance

This section explains the detailed differences between post-marketing and immediate post-marketing surveillance.
Post-marketing studies are conducted under GPSP (Good Post-Marketing Study Practice). The information that could not be obtained by clinical trials or clinical studies is collected for reexamination and reevaluation.
On the other hand, immediate post-marketing surveillance is conducted under GVP (Good Vigilance Practice) to take measures to ensure the safety of products. In other words, the investigation focuses on reports of side effects of new drugs.
Regarding the investigation period, post-marketing surveillance is from four to ten years, whereas immediate post-marketing surveillance is for six months.
During the first six months, Immediate post-marketing surveillance activities are performed once every two weeks for the first two months, and then once a month thereafter.
Issues in post-marketing surveillance
The challenge with post-marketing surveillance is that it can become a huge burden on medical institutions.
Specifically, this includes contracting for treatment outcome studies, registering clinical cases, and collecting adverse event data, such as cases of side effects.
In some cases, it is difficult to distinguish whether symptoms are caused by the primary disease or the drug.
Please contact us when conducting surveys using medical information databases
In addition to conventional post-marketing surveillance, new methods such as “A survey using medical information databases” which investigates prescribing and treatment conditions, have gradually become widespread since the revision of the GSPS ordinance.
A survey using medical information databases enables marketing authorization holders to not only confirm safety but also to use the information for future product strategies and market status research.
Medical Data Vision has one of the largest medical databases in Japan, with a total of 41.58 million patient data (as of October 31, 2022) from 473 hospitals (as of October 31, 2022), which can be used for post-marketing database surveillance.
We can also collect and aggregate patient data for your analysis and provide it as an aggregate report.
Many medical-related companies, including pharmaceutical companies and medical device manufacturers, have used our services, and we have numerous examples.
Please feel free to contact us for surveys using medical information databases.
For More Information, Please Contact Us Here
About Japanese Healthcare System

What you need to know about the healthcare system in Japan before using the data.
SERVICE

In addition to various web tools that allow you to easily conduct surveys via a browser using our medical database, we offer data provision services categorized into four types to meet your needs and challenges: "Analysis reports" "Datasets," "All Therapeutic Areas Data Provision Service," and "Specific Therapeutic Areas Data Provision Service.

© Medical Data Vision Co., Ltd. All Rights Reserved.





