The term “RMP” may sound familiar to those associated with pharmaceuticals or healthcare industry, but some may not have a clue in what an RMP truly entails.
This article delivers information of risk management plan (RMP).
What is an RMP?
In healthcare industry, a risk management plan (RMP) refers to a document submitted by pharmaceutical companies to the Ministry of Health, Labour and Welfare. It consolidates the risk management process for a pharmaceutical product, covering all stages from development to post-marketing. The RMP ensures that investigations, trials, and other measures are conducted regularly or in alignment with progress to minimize pharmaceutical risks. By creating an RMP, medical institutions can address safety risks and select appropriate medications for their patients.
The Purpose of an RMP
RMP is used to evaluate the risks, including the efficacy and side effects of pharmaceuticals, from the development stage to after-market sales. Based on the information accumulated and collected through the RMP, necessary safety measures are taken, contributing to the early detection of side effects.
In addition, RMP can be used to collect missing information, such as identifying risks that may be a concern under specific conditions, such as use in children or the elderly.
Three basic elements
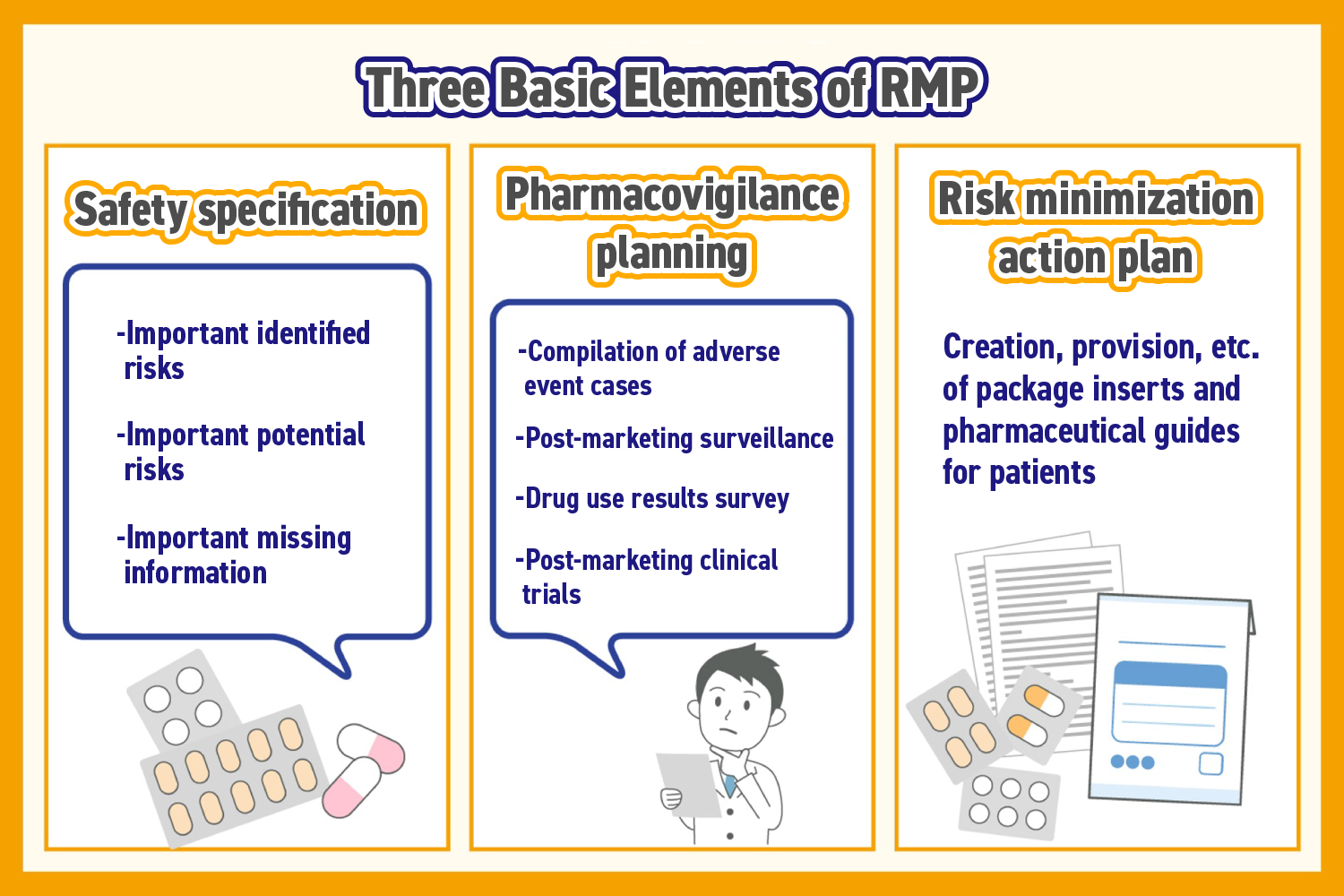
RMP is a document composed of the following three elements:
- Safety specification
- Pharmacovigilance planning
- Risk minimization action plan
The following provides an explanation of each element.
Safety Specification
The three risks listed in the RMP are collectively referred to as safety consideration.
The first is important identified risks, where there is sufficient evidence to show that the target pharmaceutical may cause harmful events. The second is an important potential risk, where harmful events are suspected to be related to the target pharmaceutical, but sufficient evidence from clinical data has not been confirmed. The third is important missing information, which refers to critical information that is lacking at the time of the RMP’s creation, and is considered important for demonstrating the safety of the target pharmaceutical.
Pharmaceutical Safety Monitoring Plan
The pharmaceutical safety monitoring plan has the nature of information collection. Activities for information collection are typically divided into routine activities and additional activities.
Routine activities include the collection of side effect cases. Additional activities include post-marketing surveillances, drug use results surveys, and post-marketing clinical trials.
Risk Minimization Action Plan
The risk minimization action plan has the nature of information provision. Like the pharmaceutical safety monitoring plan, the activities are divided into two types.
Routine activities include the creation and provision of package inserts and patient medication guides. Additional activities include providing information as part of post-marketing surveillance, distributing materials to ensure the proper use of the pharmaceutical, and setting usage conditions.
In addition, materials created under the additional risk minimization plan are marked with the Risk Management Plan (RMP) logo.
The difference between the package insert and the RMP
There is something similar to the RMP, which is the package insert, but the RMP provides broader information. The following explains the differences between the package insert and the RMP.
Explanation of the difference between the package insert and the RMP
The package insert contains information on side effects that have been confirmed during clinical trials and post-marketing for the relevant pharmaceutical. These side effects include not only serious adverse effects but also other types of side effects.
What can be understood from the RMP
Like the package insert, the RMP includes side effects confirmed during clinical trials and post-marketing, as well as important identified risks and important potential risks, such as side effects where the relationship is suspected but not yet sufficiently confirmed. Additionally, it provides information on risks where conditions for side effects have not been identified due to a lack of case numbers, such as in the elderly or children.
In addition, unlike the package insert, the RMP also specifies the reasons for identifying the risks, not just the types of risks.
How to obtain the RMP
The RMPs currently available can be viewed on the PMDA (Pharmaceuticals and Medical Devices Agency) website. You can search for the pharmaceutical you wish to view from the list of submitted items.
Additionally, you can search for the name of the pharmaceutical you wish to view from the “Prescription Medicines” banner and select the generic name from the search results to access the RMP for the specific pharmaceutical. On that page, you can also view RMP materials for healthcare professionals and patients.
The lifecycle of the RMP
An RMP is created when a pharmaceutical is newly approved for marketing or when safety concerns about the pharmaceutical arise after it has been marketed. The RMP helps with the early detection of side effects and the collection of missing information by accumulating reports related to the side effects of a specific pharmaceutical. Therefore, the RMP is regularly updated and serves as a tool for accumulating information.
Furthermore, even if the RMP is completed, healthcare professionals continue to work on minimizing pharmaceutical risks. If new safety concerns arise, an RMP may be created again through re-evaluation. In this way, the RMP is not a one-time process but can be created as many times as necessary.
RMPs lead to risk minimization
The RMP is created when a new drug is submitted for approval or when certain side effects are concerned as potential risks.
While package insert provides insights on identified risks, RMP can capture the reasons behind the risks and unidentified risks due to the lack of information, consequently contributing to the minimization of pharmaceutical risks.
Deepen the understanding of RMP is extremely crucial for capturing the benefits and risks of drugs, and minimizing risks.




