Introducing Past Case Studies using Data from MDV Database.
Case Study (Marketing)
Switch Rates, Time-to-switch, and Switch Patterns of Antiretroviral Therapy in People Living with Human Immunodeficiency Virus in Japan, using a Hospital-claim Database
Ruzicka DJ, Kuroishi N, Oshima N, et al. Switch rates, time-to-switch, and switch patterns of antiretroviral therapy in people living with human immunodeficiency virus in Japan, in a hospital-claim database. BMC Infect Dis. 2019;19(1):505. Published 2019 Jun 10. doi:10.1186/s12879-019-4129-6
Introduction
- Antiretroviral therapy (ART)
contributes to extend HIV patient’s life expectancy - Combination of the following medications have been
the recommended regimen in Japanese guidelines:
- Backbone therapy: 2 nucleoside reverse transcriptase inhibitors
- Add on of an anchor drug among:
Objectives of Study
To understand the switch rates and time-to-switch of ART regimens in patients with HIV between 2008 to 2016 in Japan
Study Design and Criteria
Study Design: Retrospective Observational Database Study.
Target Period: April 2008 – December 2016
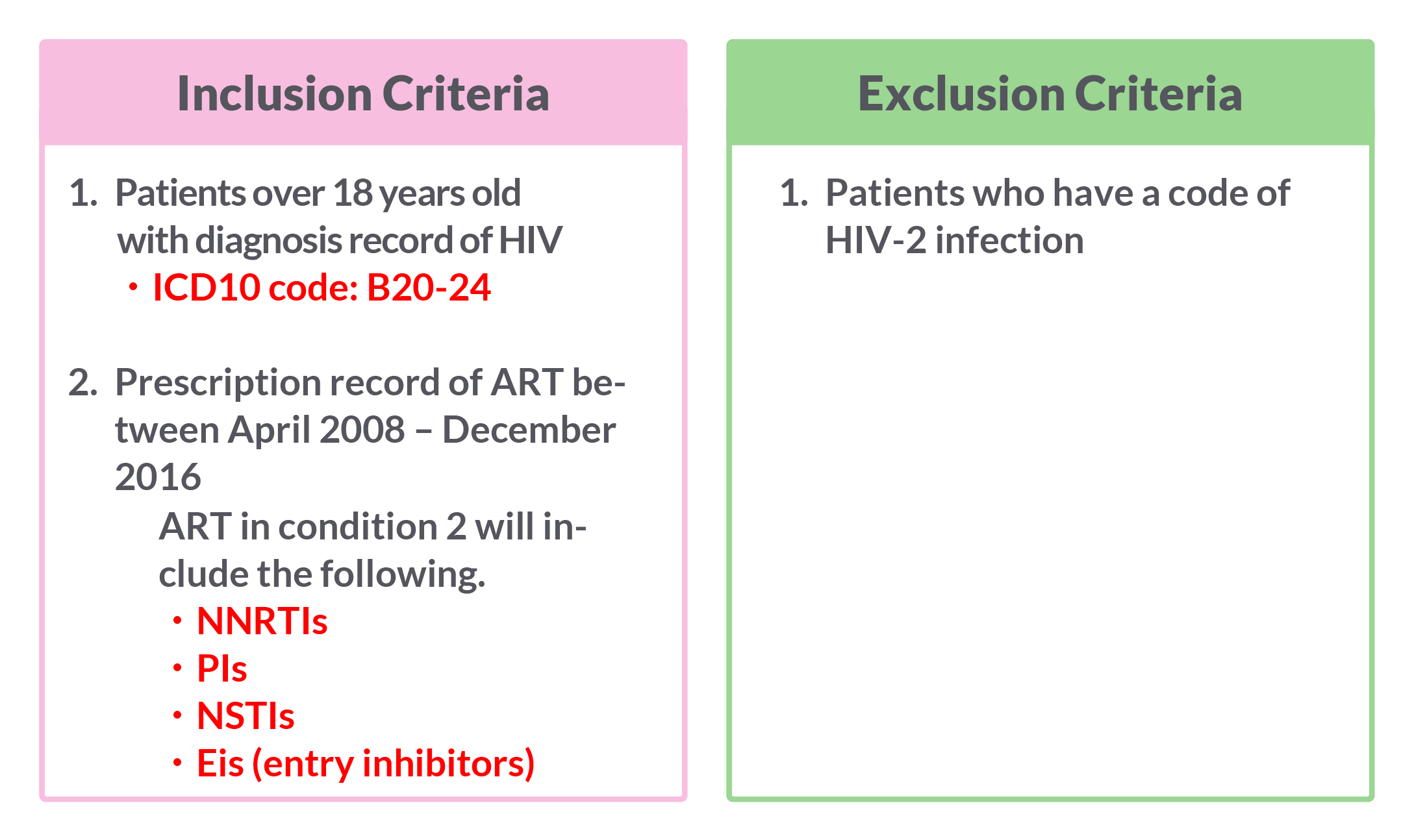
Definition of Regimen Switch
Regimen switch is referring to only when adopting anchor drug classes.
Change in anchor drug classes within 28 days of a different anchor drug is considered a change in regimen.
Patient Disposition

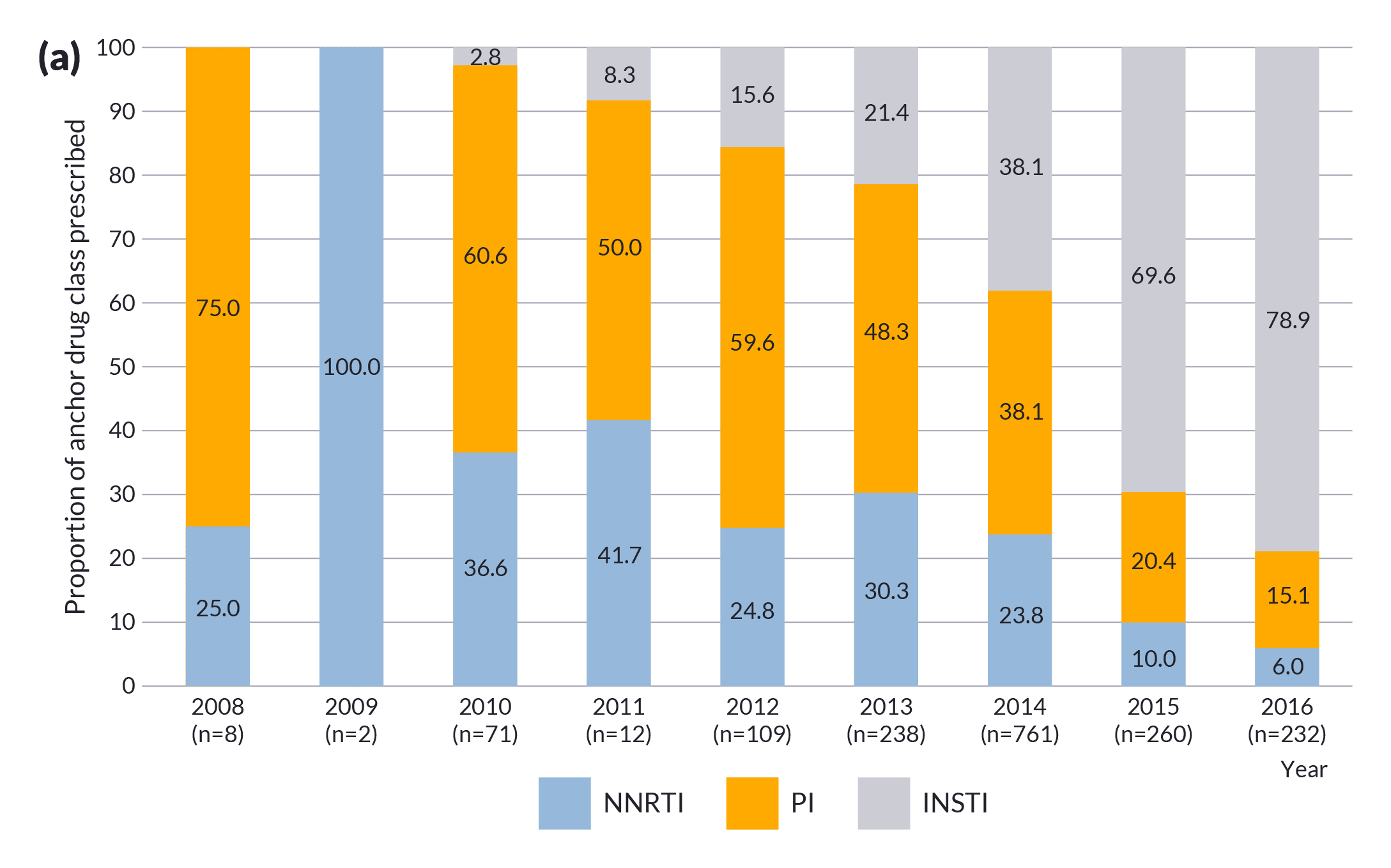
Distributions of anchor drug class and backbone in the ART regimens by year

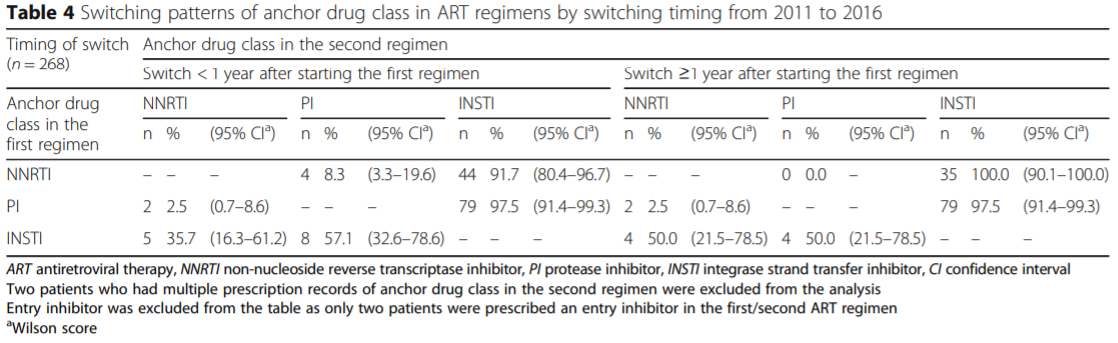
Switching Patterns and Timing
Switching patterns of anchor drug class in ART regimens by switching timing from 2011 to 2016
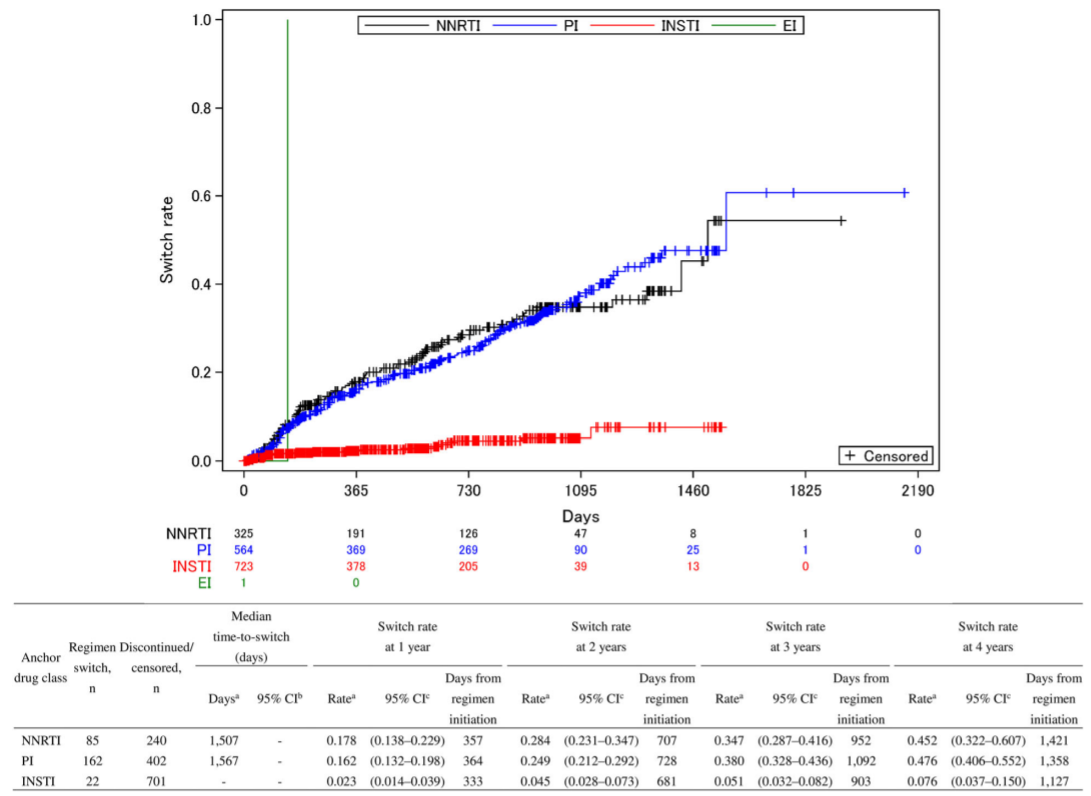
Time-to-switch
Time-to-switch of ART regimens according to anchor drug class from 2011 to 2016
Conclusion
- An ART regimen including INSTI maintained a low switch rate for long duration.
- The major switch was seen mainly from NNRTI or PI to INSTI for secondary treatment suggesting INSTI is rather a tentative treatment approach for HIV patients
Service used for the Case Study:
Abbreviation in Article
| 3TC | Lamivudine |
| ABC | Abacavir |
| AE | Adverse event |
| ART | Antiretroviral therapy |
| ATV/r | Atazanavir/ritonavir |
| AZT | Zidovudine |
| CI | Confidence interval |
| DTG | Dolutegravir |
| EFV | Efavirenz |
| EI | Entry inhibitor |
| FTC | Emtricitabine |
| HIV | Human immunodeficiency virus |
| HR | Hazard ratio |
| ICD-10 | The International Statistical Classification of Diseases and Related Health Problems 10th Revision |
| INSTI | Integrase strand transfer inhibitor |
| NNRTI | Non-nucleoside reverse transcriptase inhibitor |
| NRTI | Nucleoside reverse transcriptase inhibitor |
| PI | Protease inhibitor |
| PLWH | People living with human immunodeficiency virus |
| TAF | Tenofovir alafenamide fumarate |
| TDF | Tenofovir disoproxil fumarate |



