
Ranking of the Actual Number of Patients and Facilities for Rare Diseases in MDV Data (2022), along with the Actual Number of Patients by Observation Periods.
On February 29th of this month (Thursday), we observe Rare Disease Day (RDD), dedicated to raising awareness about rare and difficult-to-treat medical conditions worldwide. Currently, there are approximately 7,000 known rare diseases, characterized by their low patient prevalence and the complexity of their mechanisms, making progress in treatment and drug development challenging. RDD, initiated in Sweden in 2008 and in Japan since 2010, aims to enhance the quality of life for patients through improved diagnosis and treatment.
In light of this, we have extracted rare diseases from the Ministry of Health, Labour and Welfare’s using the “List of Designated Orphan Drugs (as of September 2020)” that had been approved and in which indication. We then examined the actual patient numbers and rankings of these diseases within MDV’s DPC data for the year 2022 (January to December). To complete the description of our database, we also indicated the patient population by duration of data collection. For more inquiry about MDV database applications for rare diseases, please do not hesitate to contact us.
Reference: Ministry of Health, Labour and Welfare – “List of Designated Orphan Drugs (as of September 2020)”(Japanese Only)
Table 1: Actual number of patients and number of facilities per rare disease during 2022 (January to December)
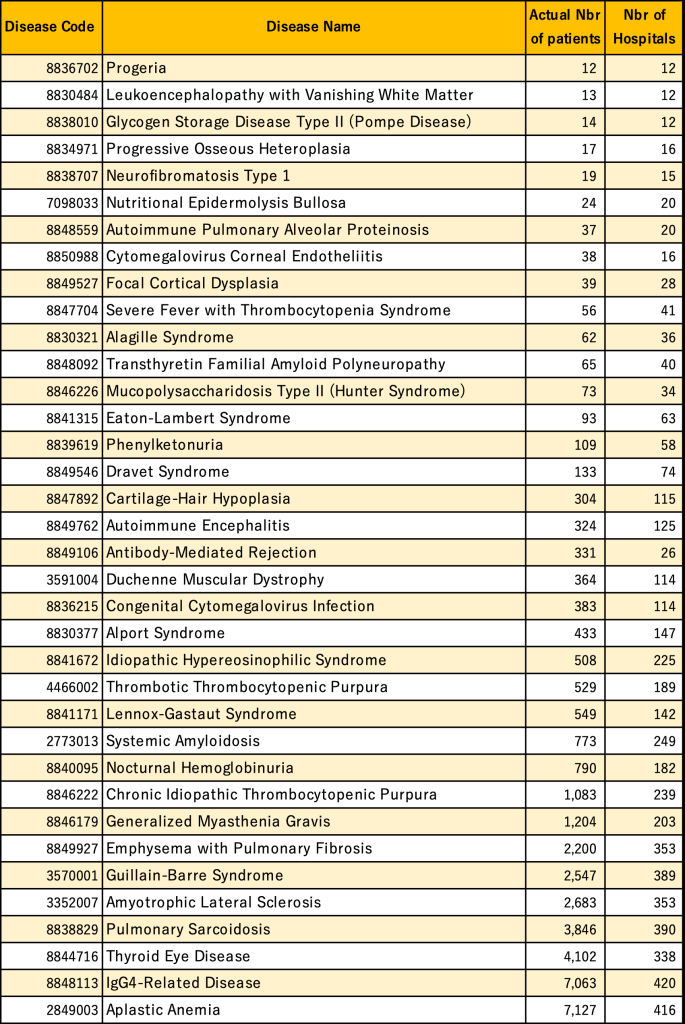
*Rare diseases with less than 10 actual patients are excluded.
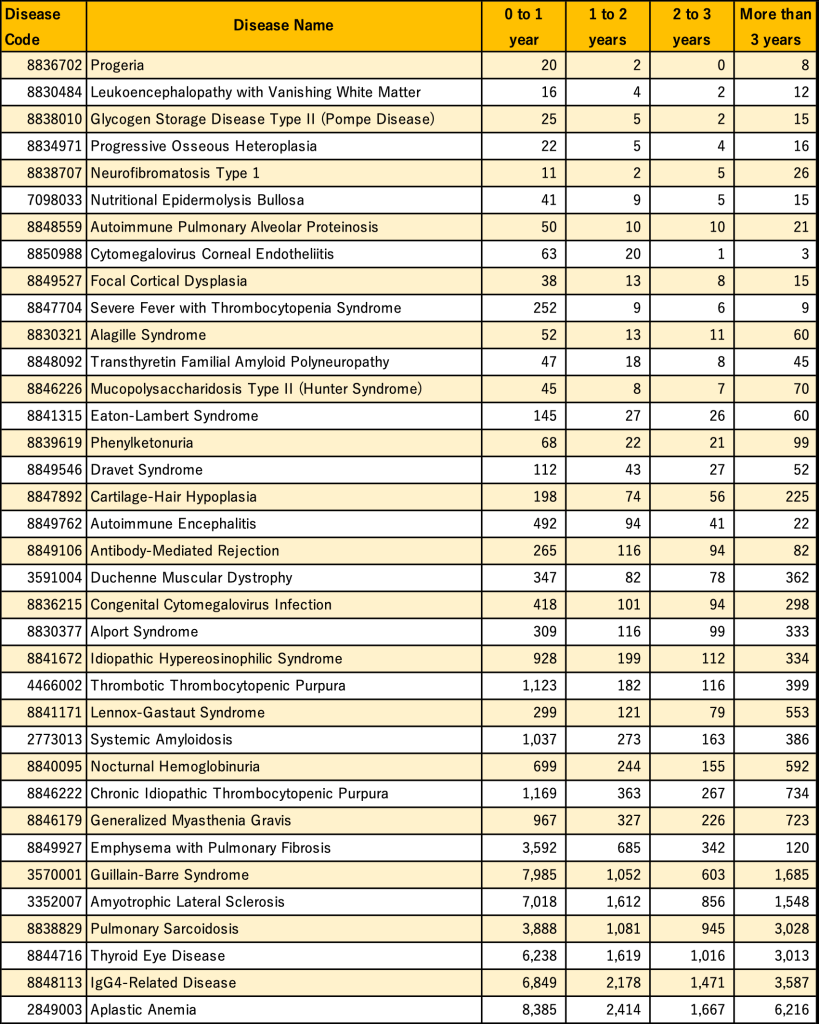
Next, we looked at the actual number of patients by length of consultation period for each rare disease as shown in Table 1 over the entire period of our data (April 2008 to October 2023).
Table 2: Number of patients by observation period for each rare disease from April 2008 to October 2023
Note: This article was published on February 1, 2024.
Data survey and analysis tailored to your specific requests
Databases, data analysis requests, and more.
© Medical Data Vision Co., Ltd. All Rights Reserved.





