What is Software as a Medical Device (SaMD)? Explaining insurance coverage standards, classification, and approval process in Japan
In recent years, Japan has seen a rapid rise in approvals of a new category of medical devices called Software as a Medical Device (SaMD). Devices such as smartwatches capable of monitoring sleep or physical activity are becoming increasingly common in everyday life and gaining popularity. Both healthcare professionals and the general public are showing growing interest.
This trend is accompanied by an increase in companies eager to develop these devices, from startups to large corporations. This article provides an overview of programmed medical devices in Japan with the latest international developments and explains insurance coverage criteria, class classifications, approval flow, and examples of medical apps.
Contents
- What is SaMD?
- Recent initiatives to accelerate approval of SaMD
- Shift in Approvals for SaMD
- The Impact of SaMD
- Examples of Approved SaMD
- SaMD Outside Japan
- Limitations of SaMD
- Development to Approval Process and Key Challenges
- Challenges from Development to Approval
- Regulatory Framework for SaMDs
- Significance of Insurance Coverage for SaMD
- Using data in development
What is SaMD?
A SaMD is a storage medium equipped with software functions that qualify as a medical device.
Medical devices are defined as products that contribute to the diagnosis, treatment, or prevention of diseases.
SaMD generally fall into two categories:
- Devices used for disease diagnosis
- Devices used for disease treatments
For example, diagnostic devices include AI-powered systems that support image-based diagnosis by learning from large volumes of medical images.
For treatment, there are therapeutic apps prescribed by doctors and installed on patients’ smartphones, with many new developments emerging.
Recent initiatives to accelerate approval of SaMD
The Ministry of Health, Labor and Welfare (MHLW) in Japan is promoting faster approval of SaMD.
As these devices are developed rapidly worldwide, Japan has been facing delays in approval, slowing local development. Domestic approval processes and practical implementation have struggled to keep pace with global demand.
To address this, MHLW has implemented initiatives considering the unique characteristics of programmed medical devices, aimed at efficient regulation. Key developments include:
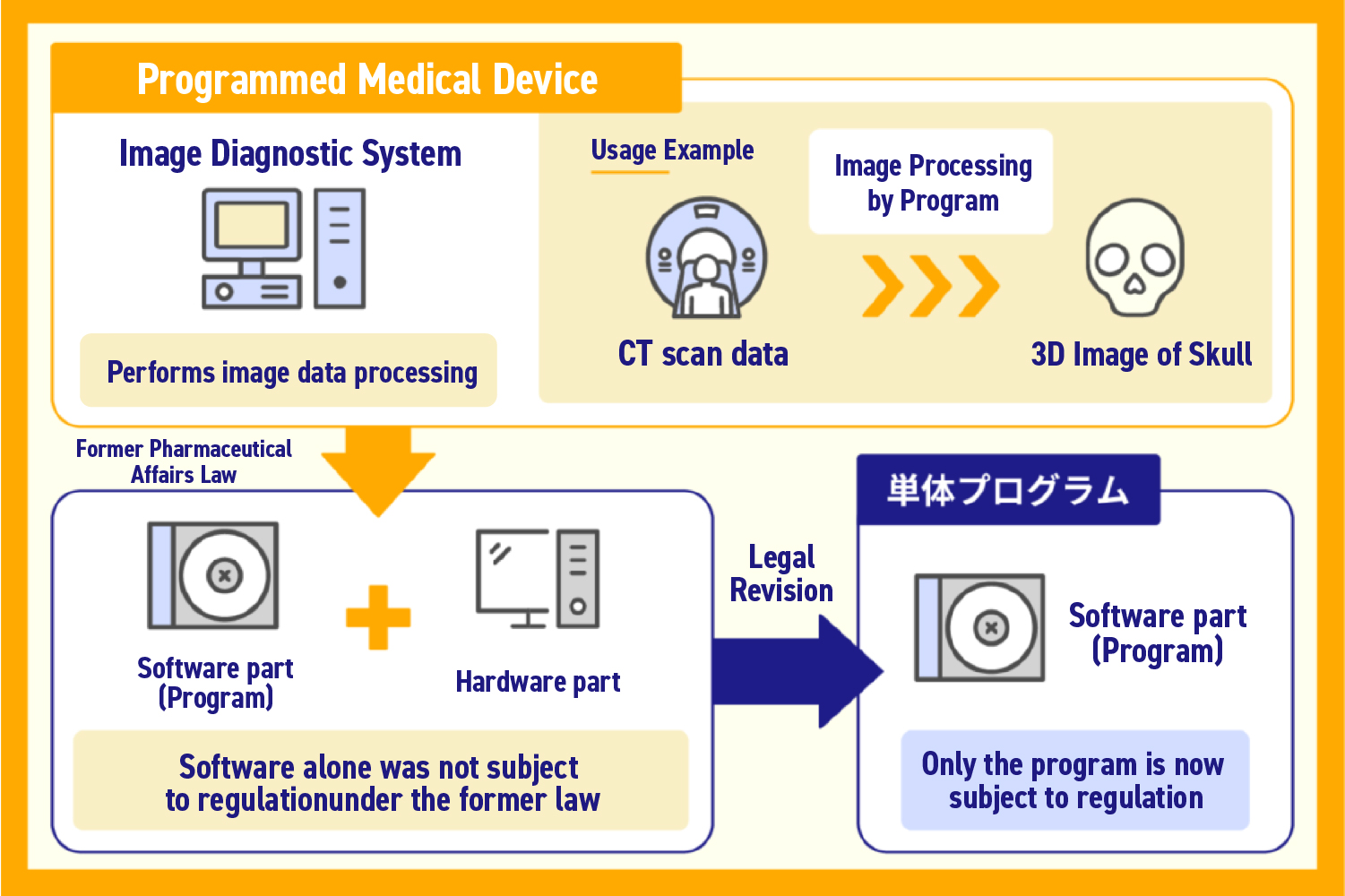
2014 : Revision of the Pharmaceutical Affairs Law, establishing independent medical device regulations
Standalone programs were explicitly recognized as medical devices, becoming subject to regulation.
2020: Introduction of IDATEN (Improvement Design within Approval for Timely Evaluation and Notice)
Addressing the frequent post-market improvements characteristic of software-based devices. Updates to apps and modifications using post-market data can now be approved more swiftly.
These include:
- Application updates
- Improvements using data collected by the device post-marketing
Accordingly, IDATEN may be called a system that is a response to this.
This has enabled changes based on post-marketing training of SaMD using AI to be approved quickly.
In addition to IDATEN, another strategy to fast-track evaluation of worthwhile new technologies was launching a system that would prioritize the evaluation of devices designated “advanced medical devices.”
Manufacturers consult with and apply to the PMDA (Pharmaceuticals and Medical Devices Agency) to receive advanced medical device certification. To be designated, the following criteria must be fulfilled.
- Innovative therapeutic or diagnostic technique
- Targets a serious disease/disorder
- Extremely safe or effective for the target disease/disorder
- Intent and framework to conduct early development in Japan first of all, and to apply for approval
2020: SaMD commercialization promotion package strategy (DASH for SaMD) enacted to promote commercialization of SaMD
Part of this system is that evaluation agencies know about new technologies in the development stage to facilitate rapid evaluation of revolutionary SaMD.
Additionally, it included the centralization of the points of contact for consultation, from development to insurance.
There are many startup companies engaging in the development of SaMD. As many of these companies are unfamiliar with pharmaceutical development, the consolidation of the points of contact for consultation is an attempt to remove a barrier to entry.
2020: First Therapeutic App Approved in Japan
Finally, 2020 also marked a historic milestone: the first therapeutic app received regulatory approval in Japan, opening the door for digital health solutions to become part of mainstream medical care.
Shift in Approvals for SaMD
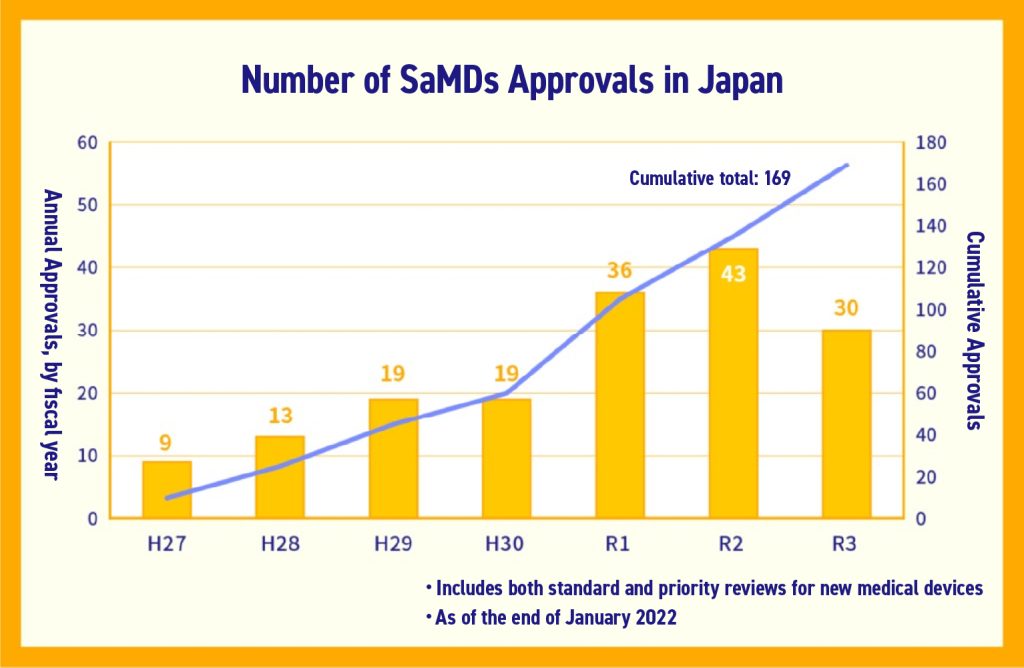
Actual approvals of SaMD are on an upward trend, with the total number of approvals at 169 as of January 2022 according to PMDA.
The Impact of SaMD
SaMD have unique characteristics compared to traditional medical devices, and they are expected to provide the following four key benefits:
1.Early Intervention in Disease
- Detecting abnormalities in the early or preventative stage of illness, and preventing worsening of symptoms by encouraging behavior modification or consulting a physician
- Reducing medical costs by preventing worsening of symptoms
2.Improved Diagnostic and Treatment Accuracy
- Improved diagnostic performance of SaMD trained on large amounts of data
- Compensating for the difference in capabilities between specialists and non-specialists
- Improved risk prediction through vitals data and surgical imaging, and safety of surgery using preoperative simulation
- Postoperative follow-up via remote monitoring
3.Increased Efficiency in Clinical Workflows
- Reducing the frequency of consultations through management using SaMD
- Shifting explanatory work to SaMD
4.Support for Education and Clinical Research
- Education and clinical research using detailed data via simulation
At the same time, concerns remain, including proper handling of personal information, ensuring AI validity, and cybersecurity risks inherent to software-based systems. Development and regulatory approval must ensure safety and reliability.
Examples of Approved SaMD
Here are some examples of SaMD that have been approved in Japan. These devices are already contributing to healthcare across a wide spectrum, from everyday wellness to advanced medical treatment.
1.Imaging support systems utilizing AI (CAD: Computer-Aided Diagnosis)
In these systems, AI trained on a large number of images, such as endoscopies, assists in determining the severity or malignancy of abnormal tissue.
There have been more than a dozen approvals for CAD systems utilizing AI, representing a large proportion of the SaMD approved.
These systems contribute to improved accuracy in diagnoses by pointing out oversights by doctors.
2.Therapy apps
Several smartphone apps have been approved for therapeutic purposes such as lifestyle diseases, smoking cessation, and neurological and psychiatric disorders.
They can intervene appropriately in disorders for which awareness, behavior, and management are important in everyday life as well as in medical examinations.
Patients who are prescribed the app track their treatment progress and daily physical condition, etc., in the app.
As a result, they are provided guidance regarding behavior modifications and treatments calculated by the algorithm in the app.
Healthcare providers can also check app usage and changes in values. These ground-breaking systems can achieve treatment results in between doctor’s visits.
3.Home diagnostic programs
These systems build an electrocardiogram based on data extracted by a wearable device, and detect abnormal waveforms and arrhythmia.
They are believed to facilitate early detection of risk factors for illness.
4.Drug schedule simulations for patients with incurable illnesses
These simulate the pharmacodynamics of drugs for individual patients with incurable diseases based on their blood sample data and symptoms.
They are intended to provide information when determining the drug dosage and dose interval.
SaMD Outside Japan
Outside Japan, SaMD are emerging and entering the market at an even faster pace. This section compares developments abroad with the situation in Japan.
SaMD which provide therapeutic interventions are known as digital therapeutics (DTx).
The world’s first DTx was a therapeutic app for diabetes patients which received approval in the US in 2010.
Conversely, the first DTx in Japan to receive pharmaceutical approval was in 2020, and by simple comparison, one can conclude there was a time lag of roughly 10 years.
Furthermore, as of 2020, it has been indicated that there is a fivefold difference in the number of approvals of SaMD utilizing AI or machine learning.
In other countries, systems have been established to fast-track pharmaceutical approval and reduce barriers to entry for development of therapeutic apps.
For example, in Germany and the US, they can receive provisional approval for insurance coverage and be prescribed to patients before the clinical results data is fully compiled, provided that they meet criteria emphasizing security and safety.
If they are able to compile their clinical results within the one-year provisional approval period, they are permitted to reapply for insurance coverage. If this is approved, then it will receive definitive registration.
Systems for promoting development are undergoing revisions in Japan as well, and it is possible that they may further reduce barriers based on the examples of other countries.
As a result of such promotion of development, SaMD have seen practical implementation in a variety of fields and specializations, including preventative medicine, treatment aftercare, and medical practice support.
In ASEAN and elsewhere in Asia, there is a great need for SaMD which facilitates everyday health management and early detection of illness, and the market is vigorous.
In this way, the market for SaMD has expanded worldwide.
Limitations of SaMD
While SaMDs offer diverse functionalities, they also have some common limitations:
- AI can be a black box, and the validity may be difficult to determine
- There is a risk of personal information leaks, and proper use must be adhered to
- Personal information collection regulations are not wholly consistent between organizations
- As there are few side effects, and they can be used easily, they tend to be used “for the time being,” and there is potential for misuse
- Possibility of cyber attacks
Development to Approval Process and Key Challenges
This section outlines the typical steps from the development of SaMD to regulatory approval, along with associated challenges. Understanding these processes in advance helps companies plan their business strategy effectively.
- Regulations for the company: business license requirements
- Regulations for the product: marketing authorization or approval
- Regulations for the manufacturing site: compliance with QMS (Quality Management System) standards
For the product itself, the approval process typically follows these steps for treatment or diagnostic software:
- Determination of classification as a medical device (MHLW or PMDA)
- If classified as a medical device, conducting clinical trials, etc. (Manufacturer)
- Pharmaceutical approval/certification (PAFSC)
- Insurance coverage (Central Social Insurance Medical Council)
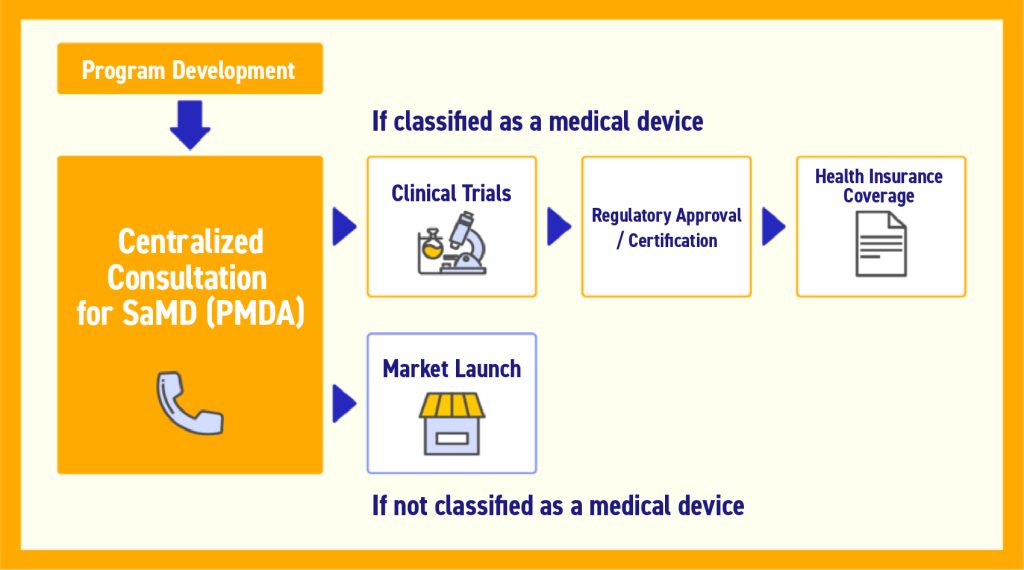
Source: 2022 Insured Medical Device System Revisions Outline (Reference Materials)
For details, please consult the guidance for approval applications published by the Japanese Ministry of Health, Labor and Welfare (MHLW).
Challenges from Development to Approval
There are several challenges in the process from the development of a SaMD to its regulatory approval.
Development Stage
- Difficult to determine classification as a medical device
- Difficult to monetize outside of insurance reimbursement, and business models vary significantly depending on classification as a medical device, making it difficult to develop a strategy in the development stage
- Personal information handling is complicated, collecting sufficient high-quality medical data is difficult
- Hurdles for conducting clinical trials are high
Pharmaceutical Approval Stage
- Approval evaluation takes time, and is difficult to forecast
- Evaluation standards are ill-defined
Marketing Stage
- Advantages of SaMD are difficult to assess under the current medical insurance system
In light of these issues, it is clear that it is necessary to clarify the objectives of the system in development and how it will achieve medical intervention early on, and to make a forecast leading up to approval.
To formulate a business plan, take advantage of General Medical Device Consultations, the central point of contact for consultations established by the PMDA, from an early stage.
Additionally, during development and approval, it is important to ensure the level of quality insurance characteristic of medical-related products.
For companies newly entering the market, it will be necessary to overcome the unfamiliar course of medical data and clinical trials, etc. Using high-quality medical data securely starting from the development stage is crucial.
Regulatory Evaluation Criteria
Evaluation criteria for regulatory approval are publicly available from PMDA, so active information gathering is important.
Key points of focus in system evaluation include:
- How much can it contribute to treatment or diagnosis? How accurate is it?
- Who is the intended target? (Healthy people, mildly ill people, severely ill people)
- How much utility can it provide to patients?
- What are the advantages for doctors?
- How great are the risks if it does not function properly?
Regulatory Framework for SaMDs
SaMD is a type of medical device. Accordingly, they are classified based on the standards for medical devices, and are subject to pharmaceutical regulations based on their classification.
The classifications of medical devices are separated based on “how much harm they cause patients when they malfunction.” The difficulty of approval and hurdles vary depending on the category of classification.
Medical devices for the purpose of diagnosis, treatment, and prevention which do not harm the patient when they malfunction are general devices.
With regard to SaMD, strictly speaking, those which “do not harm patients in the event of malfunction, and fall under general devices” are not classified as Software as a Medical Device. This means it has been determined that they do not need to receive pharmaceutical approval.
The following are the classifications for medical devices:
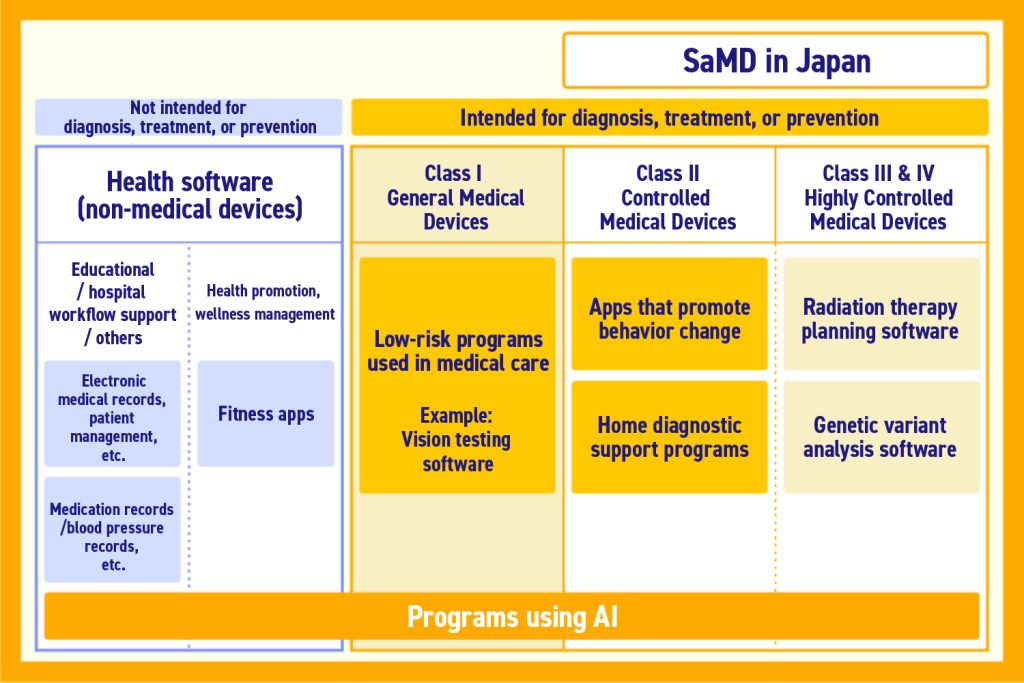
Source: Promoting Popularization of Software as a Medical Device
Occasionally, even products which have similar functions may have different classifications based on who the target is, and the extent to which intervention in treatment/diagnosis is recognized.
The business strategy will vary significantly depending on classification as a medical device, including whether or not to seek coverage by insurance and differing advertising regulations, so developers need knowledge.
Additionally, this is a transitional period in which the evaluation system for SaMD medical fees is still being actively debated.
Significance of Insurance Coverage for SaMD
In essence, coverage by insurance means being eligible for reimbursement from health insurance.
Hospitals will bear the cost when introducing the system if it is not eligible for reimbursement from health insurance, significantly limiting the hospitals to which it can be sold. Inevitably, the ability to obtain insurance coverage is very important to the business.
An issue with the existing standards for insurance coverage is the insufficient valuation of the advantages of SaMD.
For example, the merits of improving the efficiency of treatment and encouraging behavior modification are completely new benefits, so they are not adequately reflected in the evaluation standards.
The standards were also changed in 2022. However, the difficulty of obtaining insurance coverage for SaMD is still exceptionally high.
The advantages conferred on businesses and hospitals by insurance reimbursement will facilitate the popularization of SaMD, sothe establishment of standards is anticipated.
Using data in development
When developing Software as a Medical Device, it is necessary to analyze medical data (imaging and diagnostic data, etc.), and utilize it to train AI. Moreover, secure, high-quality data is essential.
Secure handling, including anonymization of personal information and obtaining consent for data collection, is necessary as well. In addition, as the accuracy of AI depends on the quality of the data, it is also important to ensure data quality.
Our company possesses one of Japan’s largest healthcare databases, with hospital data from 53 million registered patients (as of the end of August 2025) and a total of 572 hospitals.
For support in leveraging data, please feel free to contact us.
For More Information, Please Contact Us Here
About Japanese Healthcare System

What you need to know about the healthcare system in Japan before using the data.
SERVICE

In addition to various web tools that allow you to easily conduct surveys via a browser using our medical database, we offer data provision services categorized into four types to meet your needs and challenges: "Analysis reports" "Datasets," "All Therapeutic Areas Data Provision Service," and "Specific Therapeutic Areas Data Provision Service.

© Medical Data Vision Co., Ltd. All Rights Reserved.





