
Seminar
MDV & ArkMS co-hosted webinar. Database studies for GPSP compliance: real-world insights and implementation strategies.
MDV EBM Insight presents how real-world data (RWD) can be applied to healthcare research and evidence-based medicine (EBM), drawing on one of the largest clinical databases in Japan. Registered members can access a curated set of content and tools that introduce MDV’s data assets and analytical approaches. These resources are intended to support initial exploration and assessment, helping users determine relevance and potential use before moving forward with full data access. Explore the member features below and register to get started.
Videos
Access a growing library of video content, including recorded seminars on the latest trends in RWD utilization, expert panel discussions, and practical tutorials designed to support your work.

MDV & ArkMS co-hosted webinar. Database studies for GPSP compliance: real-world insights and implementation strategies.
TXP Medical & MDV co-hosted webinar. Unlocking new potential in real-world data: insights from EHR and DPC data.

Conversations with leading experts shaping the future of Japanese healthcare data.
Patient Count
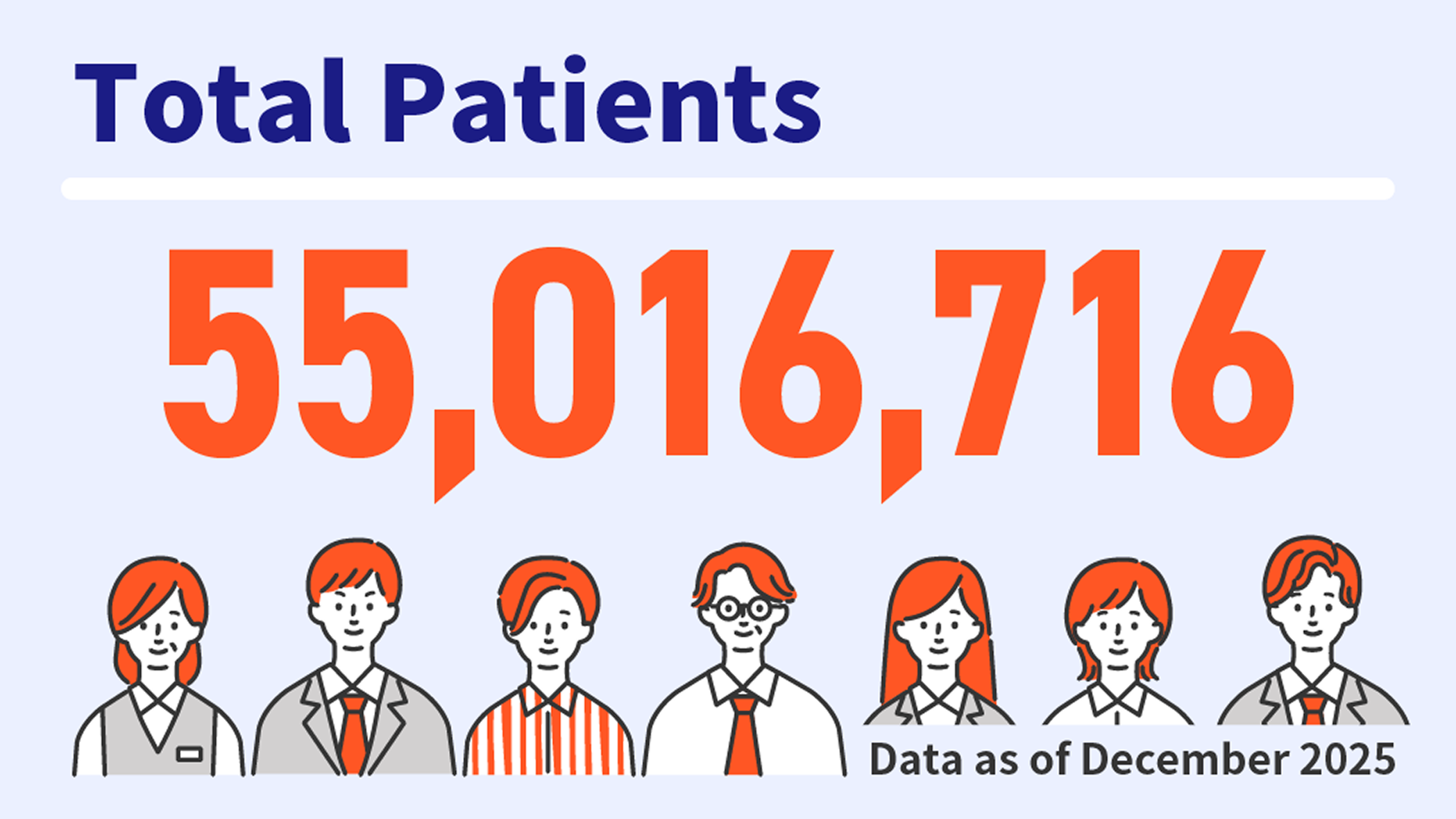
Using the patient count functionality within MDV analyzer, you can estimate patient populations by drug, diagnosis, or medical procedure. This feature is particularly useful for preliminary epidemiological studies, feasibility assessments, and post-marketing research planning.
Sample Datasets
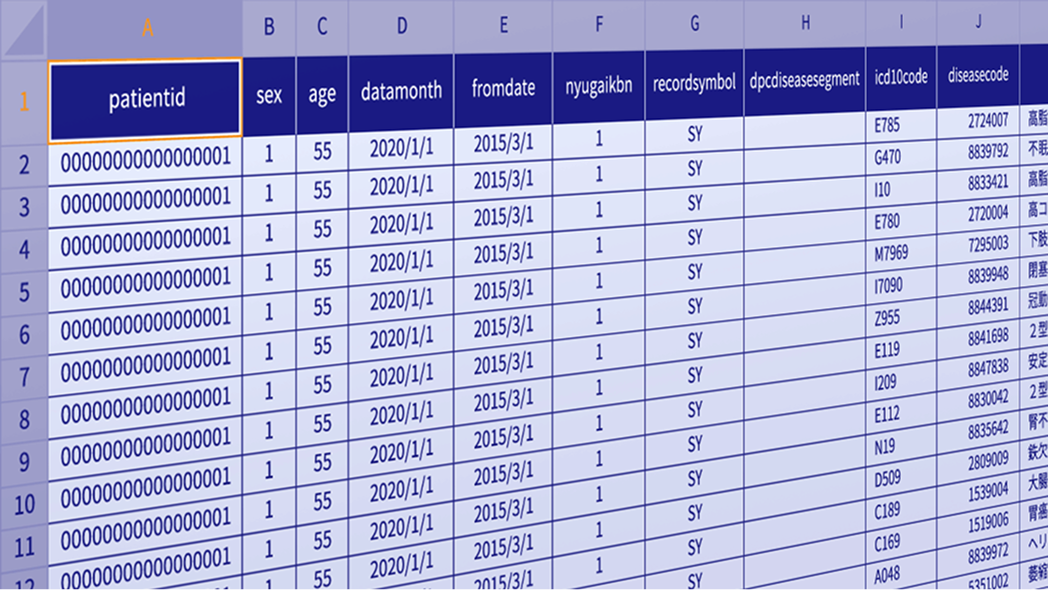
We provide sample datasets derived from MDV’s anonymized real-world data, formatted according to our standard specifications. Download the data along with documentation to review table structures and variable definitions before initiating research or system adoption.
Resource Downloads
Members can freely download a range of materials designed for pharmaceutical companies, medical device manufacturers, healthcare providers, and research institutions. These resources support effective and compliant use of healthcare big data, feel free to explore them at your convenience.
Columns
Expert perspective, insights from real-world data practice
Our columns are written by professionals with extensive experience in pharmaceuticals, medical devices, and healthcare research. They translate complex topics related to RWD utilization into clear, practical guidance you can apply in real-world projects.
Some articles are available without registration.

Explore how analysis of medical data applies to healthcare, including case studies, data analytics and practical strategies aimed at improving patient care and hospital operations.

Clear explanations of reimbursement updates and hospital-related topics for pharmaceutical field teams.

Industry trends and practical use cases in healthcare and pharmaceuticals.
Free Trial Tools
Try selected features of MDV analyzer, a suite of analytical tools designed to support market assessment, prescription pattern analysis, oncology research, and clinical decision support.
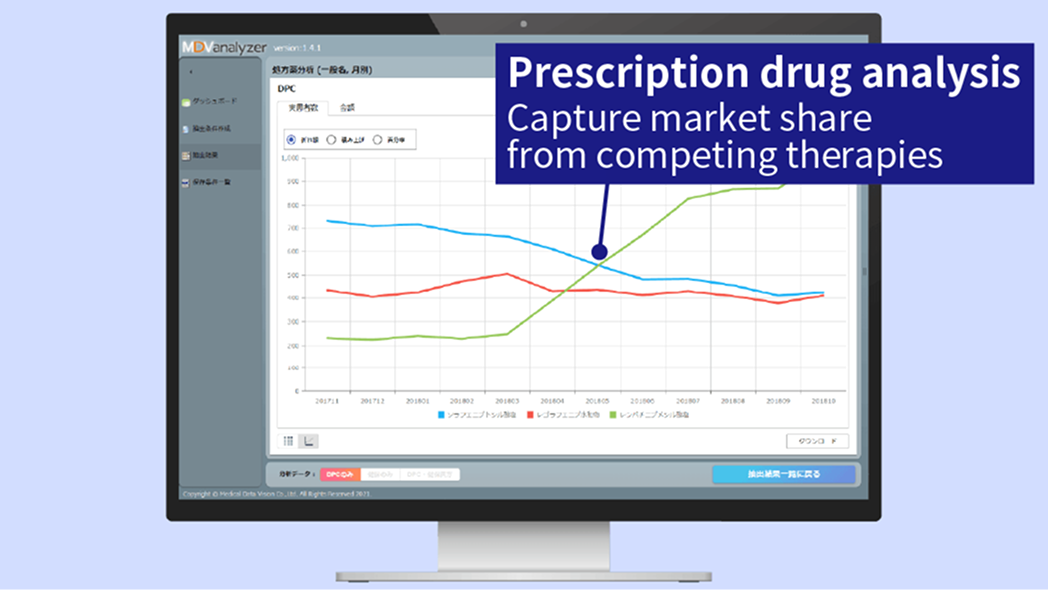

Analyze patient count, prescriptions, and clinical practices

Specialized oncology tool for analyzing regimens and lines of therapy

Support tool for research, safety reviews, and post-marketing studies
© Medical Data Vision Co., Ltd. All Rights Reserved.